Uncertainty¶
Uncertainty is probably a larger issue for geoscience than for many other natural sciences, simply owing to the fact that so much of the Earth is not physically accessible to geoscientists. A nice example of the “cost” of uncertainty is drilling for hydrocarbons in deep water. Drill ships cost ~350 000€ per day, and operating an oil platform costs ~300 000€ per day. A large, proven hydrocarbon resource is obviously worth a great deal of money, but as a geoscientist who might help decide where to drill, it should be clear that there is a big price to pay when you’re wrong.
Some general uncertainty concepts¶
It is not possible to make exact measurements. For a geologist’s compass, graduated in increments of 1 degree, the best measurement that can be made would be some degree value plus/minus 0.5°. We can say the compass needle points to a given degree, but not more precicely than within one half of a degree of that value. Even with extremely precise equipment, there will always be some uncertainty.
Our goal as geoscientists is to minimize uncertainties by making measurements that are as precise and accurate as possible (Figure 3).
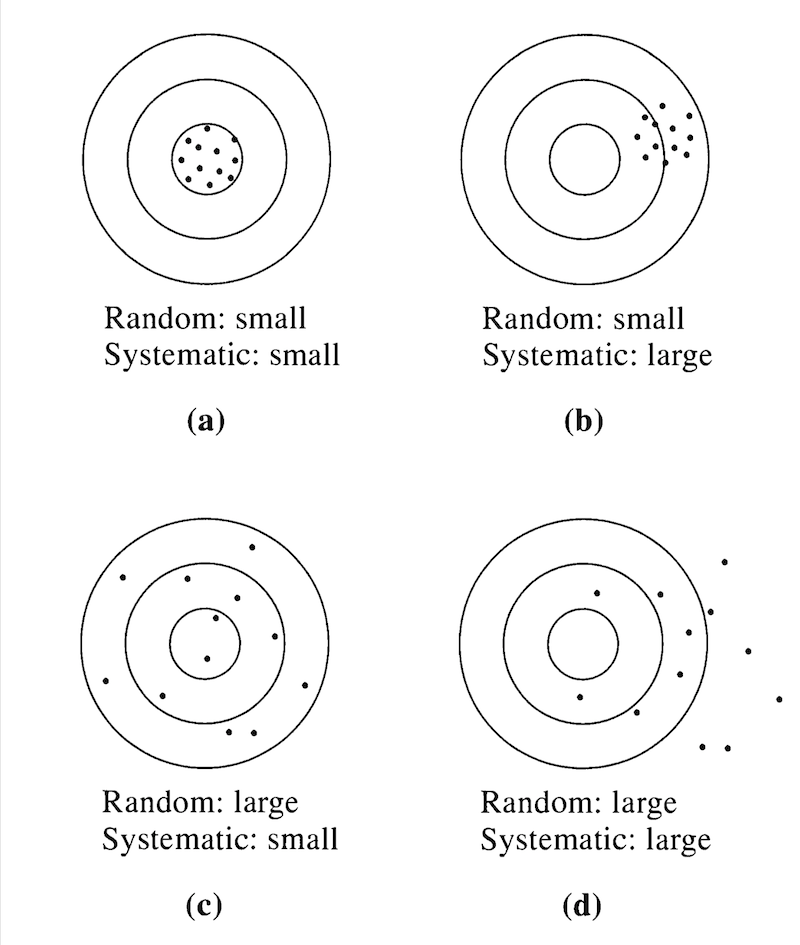
Figure 3. Random any systematic errors when the true value is known. Source: Figure 4.1 from Taylor, 1997.
Precise measurements have small random errors between measurements. Accurate measurements have small systematic errors from the “known” values. Random errors are possible to detect my repeated measurements, whereas systematic errors cannot be detected by repeated measurements (Figure 4).
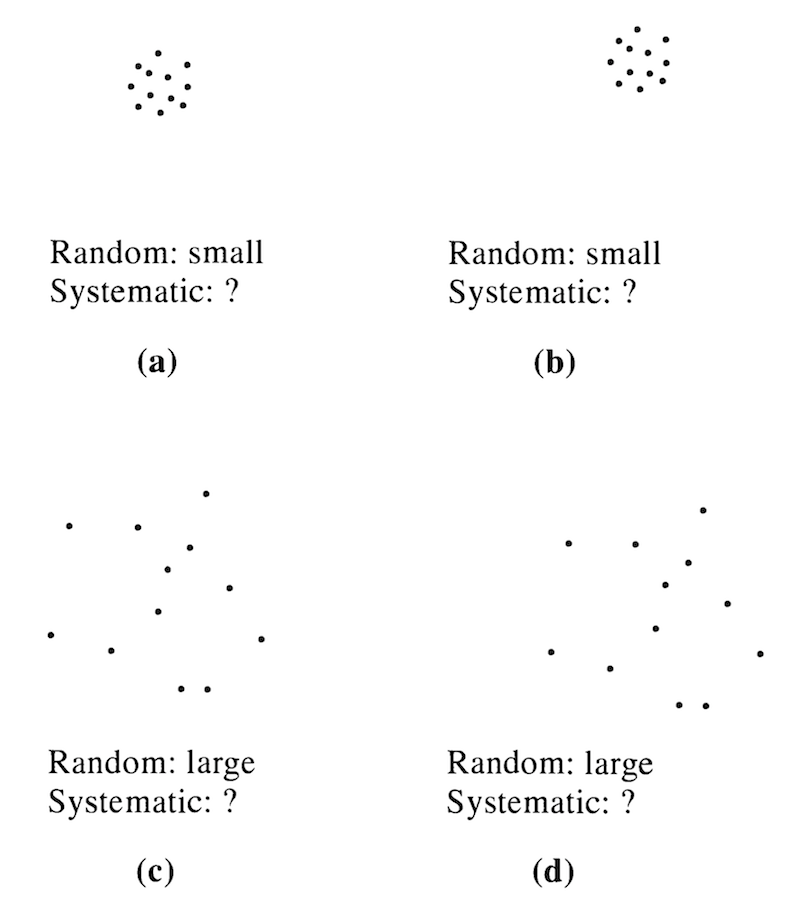
Figure 4. Random any systematic errors when the true value is unknown. Here, it should be clear it is not easy to detect systematic error. Source: Figure 4.1 from Taylor, 1997.
Systematic error might arise, for example, from a problem in the measurement equipment. This is one reason why blanks and standards are used for measuring isotope concentrations when measuring geochronometer ages, for example.